Fuel cells
Note :
As a fuel cell can be considered as a component or as a system, we will post this thematic page sheet in both sections.
A fuel cell performs the reverse electrochemical reaction of electrolysis: hydrogen and oxygen react producing electricity and water, as well as heat, according to reaction:
H2 + 1/2 O2 -> H2O
Thus we see that if the fuel used is pure hydrogen, the fuel cell only byproduct is water: this is a particularly clean generator.
The heart of the cell consists of two electrodes, the anode and cathode, separated by an electrolyte.
In some cells, such as solid oxide fuel cells (SOFC), oxide ions O2- migrate from the cathode to the anode where water is produced, and in others like proton exchange membrane fuel cells (PEMFC), cations H3O+ (hydrated protons H+) migrate from the anode to the cathode.
SOFC
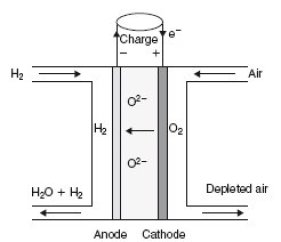
In a solid oxide fuel cells (SOFC), oxide ions O2- migrate from the cathode to the anode where water is produced.
The stack behaves as a quadrupole: In the figure below, which represents a cell stack, hydrogen enters in the top left of the stack, combines at the anode with ions O2- to form water, and exits in the lower left, enriched in water, while air enters in the top right and exits in the bottom right depleted in oxygen.
A SOFC works at very high temperatures (between 600 and 1000 °C), so that water at the outlet is in gaseous form.
Both reactions that take place are:
At the anode: H2 + O2- -> H2O + 2 e-
At the cathode 12 O2 + 2 e- -> O2-
PEMFC
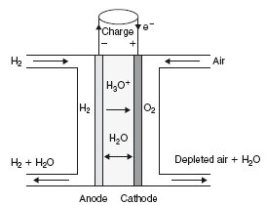
In a proton exchange membrane cell (PEMFC), hydrated protons H3O+ migrate from the anode to the cathode, where water is produced
The stack behaves as a quadrupole: In the figure below, which represents a cell stack, hydrogen enters in the top left of the stack, combines at the anode with water to form cations, and exits in the lower left , while the air enters in the top right and exits at the bottom right, oxygen-depleted and water-enriched.
A PEMFC works at low temperature (between 80 and 120 °C), so that water is in liquid form at the outlet.
Both reactions that take place are:
At the anode: H2 + 2 H2O -> 2 H3O+ + 2 e-
At the cathode: 1/2 O2 + 2 H3O+ + 2 e- -> 3 H2O
In reality, this fuel cell works only if the membrane is wetted on both sides, and is permeable to water. Water balance is governed by two phenomena: a portion of the water goes through the membrane from the anode to the cathode under the effect of electro-osmosis, driven by protons, while a part crosses in the other direction by diffusion due to concentration difference between the two sides of the membrane.
Different types of fuel cells
There are several types of fuel cells, usually classified according to the nature of their electrolyte:
AFC (Alkaline Fuel Cell) uses potash as electrolyte. Developed for space, this type of cell is used in all inhabited spaceflight at NASA, is entirely satisfactory and is cheap, but suffers from the need not operate in the absence of CO2 produced today by reforming. Its operating temperature is 80 °C;
PEMFC (Proton Exchange Membrane Fuel Cell) uses, as we have seen, a proton exchange membrane electrolyte. It seems today one of the most promising and is the subject of the largest development efforts and thereby supplants AFC. It has great potential for many applications in all capacity ranges from watts to megawatts. Its main drawback is cost, but cost reductions are hoped for in the short term. Its operating temperature is 80 °C;
PAFC (Phosphoric Acid Fuel Cell) uses phosphoric acid as electrolyte. Marketed by U.S. company ONSI Corp. for several years, it is especially interesting in CHP in light of its operating temperature of 200 °C;
MCFC (Molten Carbonate Fuel Cell) uses molten carbonate as electrolyte. Operating at high temperature (650 °C), ionic transport is performed by ions CO32-. It has the advantage of being directly fed with a synthesis gas, and can be integrated in high performance complex cycles in combination with gas turbines;
SOFC (Solid Oxide Fuel Cell) uses a solid oxide (ZrO2) doped with small amounts of calcium oxide and yttrium oxide. Its main advantage is being able to consume carbon monoxide. Operating at high temperature (600 to 1000 °C), it can also be integrated with combined cycle gas turbines.
Available Diapason sessions
Diapason sessions dealing with fuel cells are given in the table below.
n° | content | steps | soundtrack duration |
|---|---|---|---|
S60En | Overview of fuel cells | 11 | 10 mn 30 s |
S61En | 16 | 6 mn 20 s | |
S62En | 16 | 8 mn | |
S63En | 20 | 6 mn 20 s | |
S64En | Reforming for fuel cells | 34 | 17 mn 40 s |
S65En | Modeling a PEMFC | 16 | 7 mn 40 s |
Guidance page for practical work
The guidance page for practical work FG4 is dedicated to SOFC. It incorporates many points raised in the Diapason sessions S61En to S63En.
CRC_we_18
