Reduction of irreversibilities in power cycles
Types of power cycles
To convert heat into mechanical energy, in almost all the cycles used, the thermodynamic fluid is successively compressed, then heated, and finally expanded. If the cycle is open, the fluid is then evacuated to the external environment; if it is closed, it is cooled, and then compressed again.
The various engines used differ in:
the type of thermodynamic cycle they use;
the nature of the thermodynamic fluid that passes through them;
the types of hot sources;
the compression and expansion organs used.
Different typologies can thus be established, but the categories overlap partially.
Generally, there are two main categories of engines:
the first category corresponds to compressible fluid engines, where the fluid remains in the state of gas or steam throughout the cycle. For these engines, a compressor is of course necessary. Depending on the case, a boiler or combustion chamber is used as a hot source for an open cycle if the technical fluid contains oxygen (usually air);
the second category corresponds to condensable fluid engines, in which the fluid changes state. At the outlet of the condenser, it is a liquid that is compressed by a pump, then heated and transformed into steam in a boiler, steam that is then expanded in a turbine or piston engine. The compression work, proportional to the specific volume of the fluid, is in these motors much lower than in the previous ones.
We also commonly distinguish:
internal combustion engines, operating in an open cycle, for which the hot source is a combustion chamber
external combustion engines, operating in a closed cycle, for which the hot source is a boiler.
Carnot effectiveness of heat engines
Let us now turn to the Carnot effectiveness, which is very important in practice.
Sadi Carnot demonstrated in 1824 that the efficiency of an ideal thermal machine describing a cycle between two heat sources depends only on the temperatures Th and Tf cof the hot and cold sources with which it is in contact, and is given by this formula, where Th and Tf care expressed in degrees Celsius.
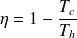
The expression of the Carnot efficiency shows that its value is all the greater the higher the higher the Th on the one hand, and the difference (Th - Tc) on the other hand is large.
The existence of a wide variety of technological solutions is mainly explained by the multiplicity of existing heat sources, the cold sources that can be used in practice being relatively few, usually ambient air, or a river.
Recall that the First Law of thermodynamics indicates that the energy of the system is conserved, while Carnot's theorem says that only the fraction
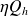 of the heat provided by the hot source is converted into mechanical energy.
of the heat provided by the hot source is converted into mechanical energy.
These two proposals may seem contradictory.
Consider a hot source with a quantity of heat Qc at temperature Tc (Figure below).

The conversion of this heat in a perfect machine exchanging with a cold source at the temperature Tf produces:
on the one hand a work W equal to
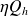 ,
,
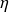 being equal to the Carnot efficiency;
being equal to the Carnot efficiency;and on the other hand a heat
 at low temperature, equal to
at low temperature, equal to
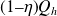
The First Law is well respected, since Qh is equal to W + Qc, and Carnot's theorem has made it possible to determine
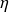 , which represents the fraction of high-temperature heat that is converted into work.
, which represents the fraction of high-temperature heat that is converted into work.
The figure below shows how the Carnot efficiency varies when the temperature of the hot source increases from 50 to 1300 °C, the temperature of the cold source being equal to 15 °C.
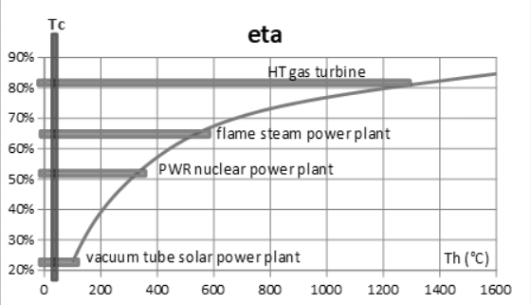
In practice, the actual efficiency is much lower than that of the Carnot cycle, on the one hand because the hypotheses which make it possible to establish this formula are almost never met, and on the other hand due to the imperfections of the machines used, which have the effect of lowering their performance because they generate what we call irreversibilities.
The differences of a real cycle with the Carnot cycle come from the following points, among other things:
first of all, in practice there must be a certain temperature difference between the machine and the hot and cold sources, which is a primary cause of irreversibility
the heat exchange with the hot and cold sources is isobaric and not isothermal, because, for technological reasons already mentioned, we generally do not know how to achieve isothermal expansion and compression
then, it is exceptional that the hot and cold sources can themselves be considered as isotherms: most often it is a fluid which exchanges heat between two temperature levels
finally, when compression and expansion are adiabatic, they are not reversible due to mechanical irreversibilities
The actual engine cycles therefore deviate significantly from the Carnot cycle.
Self-assessment activities
The following self-assessment activities will allow you to check your understanding of the Carnot cycle:
Differences between actual motor cycles and the Carnot cycle, gfe
Reduction of component irreversibilities
For various technological reasons, we donot know how to manufacture industrially components capable of both transferring heat and performing compression or expansion.
This is why compressors and expansion machines are machines in which the heat exchange with their environment is negligible, which is called adiabatic.
In addition, the work involved in a reversible adiabatic compression or expansion is, for a perfect gas, proportional to the absolute temperature Ts of the fluid to the suction of the machine.
If the indices s and rddesignate respectively the suction and the discharge of the machine, the work involved is given by this relation, f being an increasing function of the compression ratio, Ts being expressed in Kelvin and not in °C.
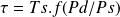
This relationship shows that the compression work is all the weaker the colder the fluid.
It is therefore always in our interest to cool a gas before compressing it, and, if the compression ratio is high and if it is technologically possible, we may be led to split the compression and cool the gas between two compression bodies, thanks to a heat exchanger.
We will see examples of this when we study the variants of the gas turbine and refrigeration machine cycles.
The reciprocal is true and it is always better to warm up a gas before expanding it.
It is therefore always in our interest to warm up a gas or steam before expansion.
Therefore, if the expansion ratio is high and if it is technologically possible, the aim is to split the expansion and warm the gas between two expansion bodies.
We will see examples of this when we study the variants of the steam plant cycles and the gas turbine.
So far, we have studied two power cycles, that of the steam plant and that of the gas turbine. We will now see how they can be improved, our goal being to minimize irreversibilities.
In practice, we will see that the changes in the cycles essentially concern:
on the one hand on the reduction of temperature differences both with the outside of the system and internally
and on the other hand on the splitting of compressions and expansions
