Representation of pure substance properties
Solid, liquid and gaseous phases
A pure substance can be in one or more of three phases: solid, liquid or gaseous. Solid state may even include several varieties known as allotropic, which reflect the different possible arrangements of the crystal lattice.
These three phases are distinguished, at the microscopic level, by the intensity of intermolecular forces. In the solid state, they allow atoms only to oscillate around fixed positions randomly distributed or ordered (crystal).
When heating a solid at a well chosen constant pressure, it turns into liquid, and we talk of fusion. If we continue to provide heat, the liquid turns to vapor, and we talk of vaporization. It is also possible that a solid turns directly into vapor, which is called sublimation. The temperature at which these changes are realized depends on the pressure exerted on the substance considered. For example at atmospheric pressure, the CO2 sublimes, that is to say, goes directly from solid to gaseous state, while water boils at 100 °C.
When a given mass of a pure substance is present in a single phase, its state is defined by two variables, for example its pressure and temperature. In the (P, T) plane, the three phases correspond to three areas, separated by three saturation curves (sublimation, vaporization and fusion) joining at the triple point T (Figure below).
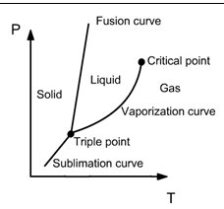
Phases of a substance
Each curve corresponds to a two-phase equilibrium. For example, the rightmost curve is the set of points representing the equilibrium of a liquid with its vapor. The two-phase equilibrium assumes that the pressure and temperature satisfy a relationship characteristic of the nature of the fluid.
For each of these phase changes to happen, it is necessary to provide or absorb energy, called latent heat of change of state. During the change of state, there are significant variations in the specific volume, except for fusion-solidification. This is particularly the case during vaporization, vapor being about 600 to 1000 times less dense than the liquid. This change in specific volume occurs at constant pressure and temperature.
Let us give some examples illustrating either the practical use of phase changes, or the constraints induced by the presence of a liquid-vapor equilibrium:
When adding ice cubes to a warm drink, we provide heat which melts them, which cools it. As the latent heat of fusion of ice is much larger than the heat capacity of the drink, we get the desired cooling effect without bringing too much water dilution;
To transport methane over long distances by sea, it is liquefied at a temperature of - 160 °C, reducing its specific volume 600 times with respect to gas. It is thus possible to maintain atmospheric pressure in the tanks of the LNG ship. Although these tanks are very well insulated, you cannot avoid some heat exchange with the surroundings, which has the effect of vaporizing a small amount of gas which is used for propulsion;
In contrast, butane or propane gas distributed for culinary purposes is confined in a liquid state at room temperature in thick metal cylinders, in order to resist the inside pressure of a few hundred psi or tens of bar;
All cooking done in boiling water takes place at 100 °C if the pressure is equal to 1 atmosphere, and this irrespective of the thermal heat supplied to the cooking. Thus we can define the precise duration for cooking a recipe, for example, a boiled egg;
The principle of the pressure cooker is to overcome this limit of 100 °C by doing the cooking in a chamber at a pressure exceeding 1 atm. It can reach 110 °C and 120 °C, in order to cook food more quickly;
An example of condensation is that which is deposited on cold surfaces in contact with moist air, like mist on a window, or the morning dew on leaves.
The triple point corresponds to the state where it is possible to simultaneously maintain equilibrium between all three phases. The critical point represents the state where the phase of pure steam has the same properties as the pure liquid phase. At higher temperatures and pressures (supercritical), it is not possible to observe a separation between liquid and gas phases: the disk surface which separates the liquid and vapor phases disappears at the critical point.
In practice, in heat engines, the working fluid is most often in the gaseous or liquid state, or as a mixture of gaseous and liquid phases. To calculate their properties, one is led to distinguish two broad categories of fluids: the ideal gas, which can be pure or compound, which includes the perfect gas, and condensable real fluids, which can also be pure or compound.
In Thermoptim's core, all these types of fluids are represented, with the exception of mixtures of real fluids, which can however be modeled by external mixtures, thanks to the TEP Lib and CTP Lib libraries.
In this portal, you will find pages dealing with:
In what follows, we will talk about liquids and solids as well as properties of phase mixtures in liquid-vapor equilibrium.
Liquids and solids
Liquids and solids are described as ideal when compressibility is negligible (v = Const).
Since an "ideal" liquid or solid cannot be subjected to any form of reversible work, a well chosen single variable is enough to represent its thermodynamic state.
du/dT = c, as for an ideal gas.
However the enthalpy of the ideal liquid (that of the solid has no physical sense) is still a function of pressure:
dh = cdT + vdP
We can still define the entropy: ds = 1/T du + P/T dv, which gives:
ds = c dT/T
As for gas, a liquid or solid whose heat capacity does not vary significantly is called "perfect": c = Const.
Then:
u - u0 = c (T - T0)
h - h0 = c ( T - T0) + v ( P - P0)
s - s0 = c ln(T/T0)
In practice, characteristics of liquids are often identified at the saturation pressure. However, as the correction v (P - P0) is generally small, this convention has little importance.
Liquid - vapor equilibrium of a pure substance
In compressible fluid machines it is often necessary to study the processes bringing the fluid into the liquid state. The ideal gas to zero does not exist, all fluids being condensable, and it is necessary to know their properties in the liquid state.
The study of vapor-liquid equilibrium is based on the law of phase mixture or lever rule that merely reflects the extensiveness of state functions with the assumption that the interfacial energy is negligible, which reads: volume, internal energy, enthalpy, entropy of a phase mixture, at pressure P and temperature T, are respectively the sums of these properties in the different phases constituting the mixture, taken in isolation at the same pressure and at the same temperature.
On various thermodynamic charts presented below, the vaporization or vapor-liquid equilibrium area is evident for temperatures and pressures lower than the critical point. This area is bounded on the left by the saturated liquid curve, and on the right by the dry saturated vapor curve. These two curves define the saturation curve, whose shape is characteristic. Between these two curves, pressure and temperature are no longer independent: they are connected by a relationship known as saturation pressure law or vapor pressure law, and the system is mono-variant.
Saturation pressure law
Many formulas have been proposed to algebraically represent the saturation pressure law. One of the most used is that of Antoine:
ln(Ps) = A - B/(C+T)
where A, B and C are characteristic parameters of the fluid, and Ps the saturated vapor pressure.
With Ps in bar and T in K, for example for water: A = 11.783 B = 3895.65 C = - 42.1387
This however is not very precise, and in Thermoptim, the following development was selected:
Vapor quality
In the middle part of the vapor-liquid equilibrium zone, fluid is present in both liquid and vapor phases. In this central zone, isobars and isotherms are combined, the liquid-vapor change taking place at constant temperature and pressure. The composition of the mixture is defined by its quality x, ratio of vapor mass mg to the total mass (mg plus the mass of liquid ml).
x = mg/(mg + ml) (16)
Enthalpy of vaporization
The length of the vaporization line gives the enthalpy (or heat) of vaporization L for the fluid conditions P and T considered. It is proportional to it in the entropy (s, T) and Mollier (s, h) charts, and equal to it in the (h, ln(P)) chart:
hg - hl = L sg - sl = L/T
The above relationships can be demonstrated from relationship 2.4.20 expressing that the free energy is minimal at equilibrium: during the vaporization process, the Gibbs energy evolves from gl to gg.
If evolution is reversible, we have: dg = 0, or gl = gg:
hl - T sl = hg - T sg
We thus find the relationship hlg = (hg - hl) = T (sg - sl) = T slg.
L is a decreasing function of temperature, zero for T above the critical temperature. A formula due to Clapeyron allows us to estimate L from the saturation pressure law:
L = T (vg- vl) dPs/dT
In this formula, the gas specific volume vg is obtained from the vapor equation of state, and the liquid specific volume vl from a proper relationship.
Calculation of pure two-phase substance properties
By applying the law of phase mixture, we have:
v = (1 - x) vl + x vg
u = (1 - x) ul + x ug
h = (1 - x) hl + x hg = hl + x L
s = (1 - x) sl + x sg = sl + x L/T
Values of critical points and vaporization enthalpies for some common substances are given in table below.
Ts (1 bar) | ro at Ts | L | Cp(Ts) | Pc | Tc | |
K | kg/liter | MJ/kg/K | kJ/kg/K | bar | K | |
air | 80.2 | 0.860 | 37.70 | 132.6 | ||
oxygen | 90.2 | 1.120 | 0.211 | 1.699 | 50.40 | 154.4 |
nitrogen | 77.4 | 0.812 | 0.197 | 2.038 | 33.96 | 126.3 |
CO2 (sublimation) | 194.7 | 0.793 | 0.369 | 73.50 | 304.2 | |
CO | 81.7 | 0.799 | 0.215 | 34.90 | 133.0 | |
water | 373.2 | 0.958 | 2.260 | 4.185 | 221.00 | 620.4 |
hydrogen | 20.4 | 0.070 | 0.467 | 9.794 | 12.96 | 33.3 |
helium | 4.3 | 0.122 | 0.023 | 4.604 | 2.28 | 5.3 |
argon | 87.3 | 1.420 | 0.163 | 1.130 | 48.59 | 150.8 |
methane | 111.5 | 0.424 | 0.503 | 3.474 | 46.27 | 190.7 |
ethane | 184.6 | 0.546 | 0.489 | 2.427 | 49.80 | 305.4 |
ethylene | 169.7 | 0.610 | 0.467 | 2.637 | 51.33 | 282.7 |
propane | 230.6 | 0.582 | 0.410 | 2.511 | 42.52 | 370.0 |
