Energy exchanges in a process
Thermal machine components are traversed by fluids that are most often gaseous or liquid. Over the processes that they undergo, these fluids exchange energy with the outside or between themselves in two forms:
mechanical, traditionally denoted W
thermal, denoted Q.
Given their practical importance, we will first study the energy exchanges.
Consistent with our bias to favor as simple as possible a presentation of the thermodynamics fundamentals, we limit ourselves in what follows to steady state systems, because even if the results we establish generalize quite easily, it is necessary to adopt a heavier formalism if we want to be rigorous..
WORK dW OF EXTERNAL FORCES ON A CLOSED SYSTEM
Consider a closed system phase. External forces acting on it are usually limited
on the one hand in the action of gravity on the fluid mass
on the other hand of pressure on its boundaries
In heat engines, the work of gravity is in most cases negligible compared to that of actions called "contact".
To fix these ideas, the work provided by a mass of 1 kg of water falling from a height of 100 m is equal to 980 J, while that of the same mass of steam at 500 °C isentropically expanded from 100 bar to 1 bar is equal to 983 kJ, or 1000 times greater.
Consequently, in thermal machines, the work of mass forces will most often be negligible compared to that of pressure.
Consider a fluid at rest (Figure below), at uniform pressure P enclosed in a cylinder whose walls are all fixed except a piston capable of moving in one direction. Now exert a force on the piston to move it slowly enough (so that at any time the system can be likened to a phase ).
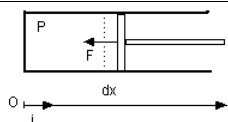
Let us consider an axis (O, i) directed from the bottom of the cylinder (x=0 and V=0). The force being exerted by the system outside, F = - ||F|| i
Call A the piston section, and dx its motion.
Obviously ||F|| = P A, and work m dWA received by the mass m of fluid is:
m dWA = - ||F|| dx = - P A dx = - P dV.
dV being the volume change of the mass, equal to mdv, and:
dWA = - Pdv
In case of compression, the volume change is negative and the work received is positive. We note this work with index A, to indicate it is exercised on the surface of the system considered.
This expression, which generalizes easily to a closed system of any form, is valid only if the system remains in static equilibrium, i.e. if the following assumptions are true:
the pressure remains uniform across the system;
the fluid remains at rest.
Note that formula (1) implies that the work received by the system is positive, and that the work it provides is negative. By convention, we generalize this result by counting positively the energy received by a system, and negatively the energy it provides to the outside.
During a reversible process making a closed phase pass from state 1 to state 2, the mass work of contact actions has the value:

In case the gravity work is not negligible, one can easily show that it is expressed as:
dWv = -g dz (3)
g > 0 is the acceleration of gravity, and z the altitude of the point, counted positively upwards. We note such work with index v, to indicate it is exercised in the volume of the system considered.
We have then:
W = WA + WV (4)
HEAT TRANSFER
Consider a simple fluid mass, within which no friction occurs. dQ is the quantity of heat exchanged with the outside and received by the unit of fluid mass in an infinitely small process.
An important experimental fact, the basis of compressible fluid thermodynamics, is that dQ is a differential form of the fluid mass state, called a calorimetric equation.
For example, in the case of a mono-variant system (equilibrium between phases during a change of state), the calorimetric equation depends only on the quality x, ratio of vapor mass to total mass (liquid + vapor) in the case of vaporization or condensation:
dQ = L dx
L is the change of state enthalpy.
More generally, the calorimetric equation that connects dQto the simple fluid (bi-variant) state variables can take three well known equivalent canonical forms:
dQ = cp dT + h dP
dQ = cv dT + l dv (5)
dQ = m dP + l dv
Coefficients cp, cv, h, l, m and l are called equilibrium fluid calorimetric coefficients (note that h is not enthalpy, which will be introduced later).
They are linked by differential relations that can be obtained without significant difficulty, but which do not interest us. Just know that the calorimetric properties of a fluid are determined by knowing two of these six coefficients.
Subsequently, we will use only coefficients cp and cv, which are respectively called specific heat capacity at constant pressure, and specific heat capacity at constant volume, whose former name was "specific heat".
For a solid or a liquid, cp = cv, and dQ = c dT
For an ideal gas, h = l = 0, and, depending on whether we are dealing with an open system operating at constant pressure, or a closed one operating at constant volume, dQ = cp dT or dQ = cv dT. For a real gas, use one of the canonical forms above.
The above equations are however valid only if certain conditions are met:
Firstly, the mass of fluid must be completely homogeneous, i.e. comparable to a phase;
Secondly, we assumed that there was no irreversibility inside or at the boundary of the fluid mass.
If any, the relationship becomes: dQ < cp dT + h dP
We will then write: dQ = cp dT + h dP - dp (6)
dp, essentially a positive term, has a very simple physical meaning: it is the heat generated by mechanical friction within the fluid. Although it differs profoundly, it produces the same effect as heat received from the outside. Its meaning will be clarified during the presentation of the Second Law of thermodynamics .
By convention, therefore, we denote dQ the heat exchanged with the outside, and counted positively if received by the system, and dp the heat dissipated by internal friction if any.
In practice, it is important to distinguish these two forms of heat, otherwise serious reasoning errors can be made. In particular, the processes without heat exchange with the outside, called adiabatic, are such that dQ = 0, , whether or not the seat of irreversibility, that is to say dpbeing either zero or not.
