Modelling of associating fluids
Introduction
In this page, we introduce new EoS which were recently proposed to overcome the limitations encountered by the cubic equations of state. They are based on developments in statistical thermodynamics of fluids. Among those which today are very popular, especially for so-called associating fluids containing hydrogen bonds as water, methanol and hydrocarbons, let us mention the Cubic Plus Association (CPA) model, which adds additional terms to the a cubic equation, and the family of Statistical Associating Fluid Theory (SAFT) models.
The CPA equation adds an association term to a Soave Redlich Kwong type (SRK) or Peng Robinson (PR) EoS, while maintaining the characteristics of the cubic EoS for non-associating molecules, which significantly improves their predictive ability.
The SAFT theory was developed from 1989 based on the work of Chapman, who proposed a statistical theory for the thermodynamic characterization of a fluid by incorporating the effects of association and the various interactions taking place between its molecules. It provides excellent results for modeling the behavior of associating fluids and non-associating chains.
The main advantage of the SAFT theory is that it relies on a more realistic representation of the physical molecules, taking account of their shape, size, and the various interactions that can exist between the molecules of a fluid. Furthermore, unlike cubic EoS, the SAFT equation may represent non-spherical molecules and different types of fluids (electrolyte solution, polar solvents, fluids with hydrogen bonds).
The physical significance of the SAFT equation parameters makes possible the development of a group contribution method for their determination. A version simpler than others is the PC-SAFT (Perturbed Chain Statistical Associating Fluid Theory) equation, developed by Gross and Sadowski, considered reliable in the field of modeling of associating and oxygenated compounds.
Models of statistical thermodynamics
These models being based on statistical mechanics, a few reminders may be useful to fully understand the fundamentals. From a microscopic description of the phenomena, the kinetic theory of gases allows one to find their macroscopic properties (volume, enthalpy, entropy, transport properties).
The simplest model is the one where it is assumed that the size of molecules and the interactions between them are negligible. It leads to the ideal gas law:
PV = RT
The ideal gas theory is based on the assumption that gas molecules behave as hard spheres of negligible size, that is to say dimensionally stable, which collide with each other and elastically bounce without any other interaction between them.
In an ideal gas, we consider that the molecules do not have any volume and only have a kinetic energy due to their movement speed. This model mainly applies to low-pressure gases, far from their saturation curve.
One of the major interests of the ideal gas model, in addition to the simplicity of its resolution, is that a mixture of ideal gases behaves itself as an ideal gas, as stipulated in the law of Dalton.
It should be noted that an ideal gas is a perfect gas whose specific heat Cp (or specific heat capacity) varies depending on the temperature.
Unlike the ideal gas, the molecules of real fluids interact with each other through specific interactions. A molecule in the vicinity of a second molecule is subjected to attractive and repulsive interactions, whose intensity depends on the intermolecular distance between them, on their orientation, and on their atomic structure.
Considering the size of molecules, one can describe the collisions between them, and obtain the transport properties (viscosity, thermal conductivity, etc.).
The attractive forces are divided into two types: physical and chemical. It is the latter which ensure the cohesion of the molecule.
The physical forces are of electrostatic nature and occur over long distances. London forces (dispersion) are proportional to the value of the polarizability and the ionization potential of the molecule. Polar molecules have electric dipole which introduces additional interactions, such as Keesom forces (dipole-dipole), and Debye forces (dipole-induced dipole).
The van der Waals forces or intermolecular forces correspond to interactions between molecules at short range (between 3Å and 8Å). They do not alter the nature of the molecules but produce, on average, a total force of attraction responsible for the cohesion of the material in condensed media.
At very short distance (less than 3 Å), these interactions become repulsive and they prevent the molecules to approach completely. The attractive and repulsive forces balance at an average distance called van der Waals radius.
Taking into account the interaction forces between the molecules, the van der Waals equation below is used to describe the liquid-gas transition (boiling, evaporation, condensation), but with limited accuracy:
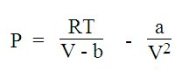
The van der Waals equation includes two terms, one attractive and the other repulsive, based on the model of hard sphere:
a, attraction parameter is a measure of the strength of attraction between molecules;
b, called covolume, represents the part of the molar volume that is not actually available because of the presence of other molecules.
Equations of the type Soave-Redlich-Kwong (1972) and Peng-Robinson (1976) are cubic EoS more accurate than the previous one. They are widely used in applied thermodynamics. They consider that the molecules are spherical or slightly aspherical.
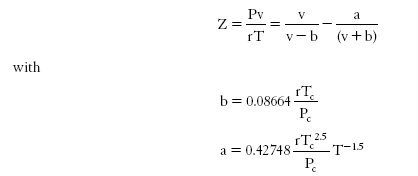
However, these EoS are only applicable for molecules assumed to be spherical and which do not associate. So they have their limits when used to represent a fluid whose molecules are highly non-spherical or that can associate.
By introducing additional concepts presented below, new theories derived from statistical mechanics helped develop equations based on the molecular behavior of fluids, which are capable of exceeding certain cubic EoS limits.
To understand these new equations, we must first clarify the meaning of certain concepts:
chains of molecules;
potential functions;
hydrogen bonds,associating fluids and association sites.
Chains of molecules
Many molecules are far from being spherical, but appear to consist of atom groups, referred to as spherical segments, that can be considered as organized chains.
The concept of chain of hard spheres allows one to develop equations of state such as SAFT to represent polymers or complexes (amines, alcohols, acids). Statistical mechanics makes it possible to model them as assemblies of spherical segments forming chains.
Potential functions
The van der Waals forces result in what is called the intermolecular interaction potential. To represent this potential several types of models have been proposed, including those listed below:
Lennard-Jones interaction potential model;
square well potential interaction model.
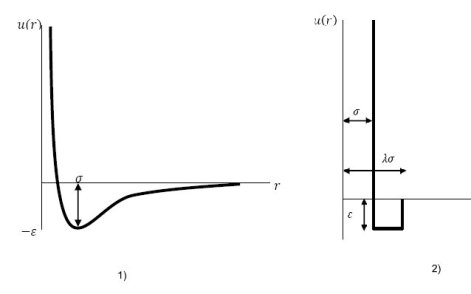
In thermodynamic models of statistical mechanics, interactions between molecules can be represented by these simplified potential functions that, used with a radial distribution function, allow one to determine the main thermodynamic properties of fluids, be they pure substances or mixtures.
The radial distribution functions are complex and cannot be solved easily for the most accurate intermolecular potential functions. Using perturbation theory, the problem can be reformulated as a development of the Helmholtz free energy, leading to a non-cubic general form of the equation of state from which the free energy can be expressed. This approach is implemented in the CPA and SAFT models.
Hydrogen bonds and associating fluids
Hydrogen bonds are intermolecular forces existing between a hydrogen atom and an electronegative atom such as oxygen, nitrogen or fluorine.
The hydrogen bonding is considered as a chemical strength or chemical equivalent, whose intensity is several orders of magnitude greater than the physical forces and an order of magnitude lower than that of chemical bonds. For example, the interaction energy of van der Waals forces for methane is 0.6 kJ / mol, while that of the hydrogen bond of water is 22.3 kJ / mol, and that of an OH chemical bond is 465 kJ / mol.
Hydrogen bonds allow the formation of complex molecules and polymers, to which they impart specific behaviors.
For example, in the absence of hydrogen bonding, the boiling temperature of water at atmospheric pressure would be 80 °C instead of 100 °C, and its melting point - 110 °C instead of 0 °C.
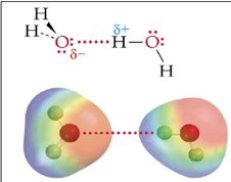
The notion of associating fluid also requires to be well defined. Associating fluids can be described as fluids having an ability to form hydrogen bonds. Associating fluid molecules combine to form long chains of polymers or dimers. The intermolecular forces involved in these fluids are intermediate between firstly the dispersion forces or weak electrostatic interactions, and secondly the forces characteristic of chemical reactions forming the molecules.
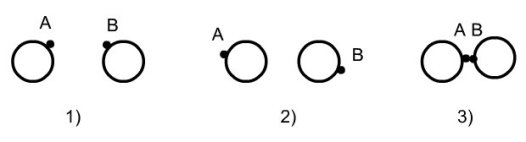
Associating fluids exhibit one or more association sites, each of them being characterized by a potential placed near the perimeter of the molecule. Associating interactions depend on the distance and orientation of the molecules. The figure below shows an example of spherical segments equipped with association sites A and B. The two spheres can form AB dimer bonds only if the distance and orientation of the sites are favorable:
molecules too far away: no association;
misdirection;
association achieved.
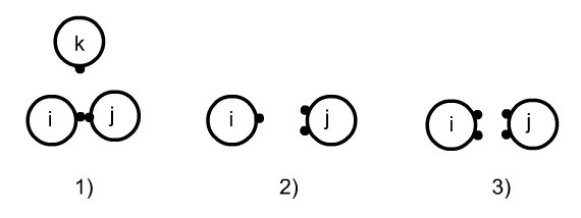
Association rules are described in the figure below:
two sites cannot associate with a third;
a site of the molecule i cannot associate simultaneously on two sites of j;
double association is not permitted.
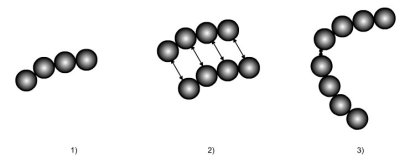
The figure below illustrates the concepts just mentioned:
a complex molecule is modeled as consisting of four segments of hard spheres;
the interaction forces between two remote molecules are represented;
an associating link between two sites links two chains.
The association function via a square well potential can be characterized by two values, the energy of association (depth of the well) and a parameter characterizing the volume of association (bound to the well width).
The CPA equation adds to a cubic EoS an association term characterized by two parameters, epsilon and beta.
In addition to these values, the association scheme must be specified, that is to say the number of sites and their type. This choice requires skills in chemistry beyond the scope of this page.
Based on these concepts, new theories derived from statistical mechanics lead to the development of the SAFT or CPA EoS able to accurately represent the thermodynamic properties of fluids, be they associating or not, complex or polar molecules.
These new equations of state can be used to model, with a small number of parameters, some refrigerants whose molecules are usually polar but not necessarily associating.
Results
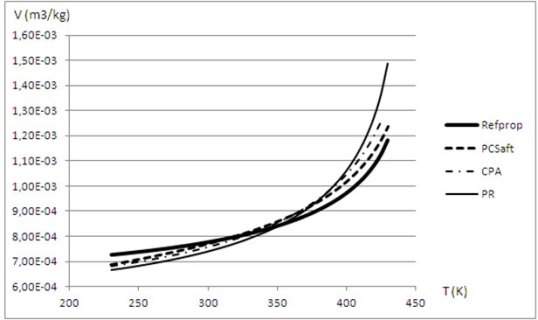
The results provided by these models are shown in the figures below, which give the liquid volume of water and R1233zd calculated using different equations of state.
For water, the reference values, in bold solid line, are those of the IF-IAPWS 97. The SRK equation, even with adjusted coefficients fails to provide acceptable values: the calculated volume (thin line) is always greater than the reference value.
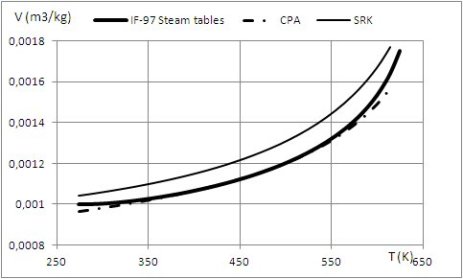
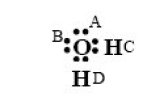
In contrast, the CPA model, in dots and dashes, is excellent, thanks to the association term: the CPA model considers that the water molecule has four association sites among which two electron acceptor protons C and D (so-called scheme 4C), as shown in the figure below.
The reference model for R1233zd is REFPROP, bold solid line. The equation of Peng Robinson (PR, thin line) is the one that deviates the most. The CPA model (without association term) which is in this case simply a cubic EoS with adjustable parameters, is a bit better (in dots and dashes), but the PC-SAFT model (without association term) gives the best values.
References
Chapman W.G., Gubbins K.E., Jackson G., Radosz M., (1990), New reference equation of state for associating liquids, Ind. Eng. Chem. Res., 29, 1709-1721
Kontogeorgis G.M., Voutsas E.C., Yakoumis I.V., Tassios D.P., (1996), An equation of state for associating fluids, Ind. Eng. Chem. Res., 35, 4310-4318
Kontogeorgis G.M.,Folas G.K., Thermodynamic Models for Industrial Applications, From Classical and Advanced Mixing Rules to Association Theories, Wiley, 2010, ISBN 978-0-470-69726-9
Gross, J., Sadowski, G. Perturbed-Chain SAFT: An Equation of State Based on a Perturbation Theory for Chain Molecules. Ind. Eng. Chem. Res. 2001, 40, 1244-1260
