Boilers
There are two main types of boilers, known from the fluid that circulates inside the tubes: fire tube boilers, and water tube boilers.
In the first, the flame develops in a corrugated tube, then the flue gases pass inside tubes, in one or more passes, water being at the outside;
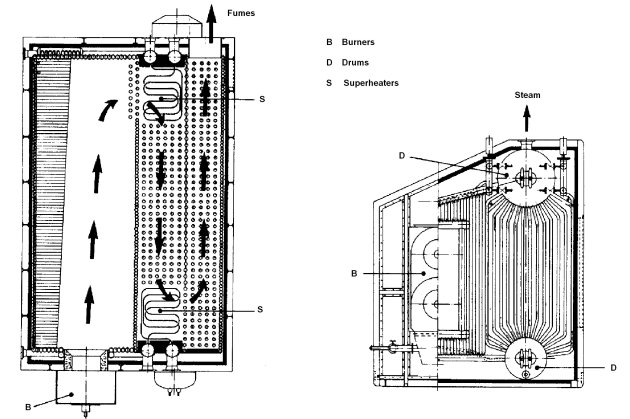
Within the second type, water circulates by natural or forced convection between two drums placed one above the other, through a network of tubes. The flame develops in a furnace lined with tubes that absorb the radiation. A second tube bundle receives heat by convection from the flue gases. The water rises in the tubes subjected to radiation, and falls by the convection assembly.
Fire tube boilers

Water-tube boilers
The fire tube boilers can achieve flue gas temperatures lower (220 to 250 °C) than water-tube boilers (300 °C) without an economizer, which gives them a slightly better efficiency.
However, the former are limited to capacities lower than the latter, for reasons of mechanical strength and safety (with a very large volume of water under pressure).
Their main area of use is the supply of saturated steam under low pressure (<15 bar), and represent over 60% of the French fleet of boilers, against 20-25% for water tube boilers, which are well suited for the supply of superheated steam at medium and high pressure.
A boiler has three successive functions:
heat pressurized feedwater (in the economizer) to the vaporization temperature corresponding to the pressure;
vaporize steam;
and finally superheat steam at the desired temperature.
Thermodynamic characteristics
As a first approximation, boilers can be considered as isobaric . The pressure drops are in fact generally relatively low.
When the combustion is not stoichiometric, it can be characterized in several ways:
either by excess air e, which as its name implies, represents the amount of air in excess
or by the air factor lambda, which is the term multiplying the air in the combustion equation
or by the richness R, ratio of the number of moles (or mass) of fuel in a quantity of mixture, to the number of moles (or mass) of fuel in the stoichiometric mixture.
For stoichiometric mixture lambda = 1, with excess air lambda > 1, with an excess of fuel (lack of air) lambda <1
These three variables are linked by simple relationships: lambda = 1 + e, and R = 1/lambda
In Thermoptim, a boiler is represented either by a combustion chamber represented by the icon:
or by a triple interchange involving exchange processes represented by the icon:
The setting of combustion screens is done using lambda.
the first is to give the value of lambda and calculate the end of combustion temperature and the flow-rate of fuel required
the second is to give the value of the end of combustion temperature and to calculate lambda and the flow-rate of fuel required
the third is to give the value of the fuel flow-rate and to calculate lambda and the end of combustion temperature
Book reference
An excerpt of the textbook chapter is freely downloadable with the agreement of CRC Press
Available Diapason sessions
n° | content | steps | soundtrack duration |
|---|---|---|---|
S15En | 16 | 9 mn 50 s | |
S16 | Technologie des chambres de combustion et des chaudières | 9 | 4 mn |
