First law of thermodynamics
This session is a course reminder, and a brief discussion of energy forms put into play and the first law of thermodynamics..
It shows the different terms that appear in the expression of the first principle in steady state, in closed or open system.
The three links below provide access to long excerpts of the textbook, to which you should refer. The first document outlines the basic concepts and various definitions used, and the two others are written materials on this session.
Course reference:
- “Fundamentals of Thermodynamics / Energy put into play”
- “Fundamentals of Thermodynamics / First law”
To follow the presentation, go to next step
- textbook: definitions (pdf 235 Ko)
- textbook: energy exchange (pdf 224 Ko)
- textbook: first law (pdf 283 Ko)
THERMODYNAMICS FUNDAMENTALS
- Energy exchange with the surroundings: 2 forms
- heat δQ (on the system boundaries)
- work δW:
- pressure forces δWA (on the boundaries )
- gravity δWv (in the system volume, negligible)
THERMODYNAMICS FUNDAMENTALS
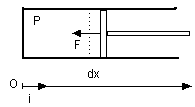
- Work δW, by action of pressure forces on the boundaries
- mδWA=-PAd
- (2.2.1)
- (2.2.2)
- (2.2.3)
THERMODYNAMICS FUNDAMENTALS
- Shaft work τ (open system)
- (2.2.5)
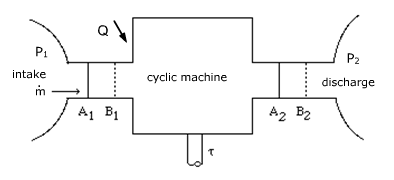
THERMODYNAMICS FUNDAMENTALS
- Shaft work τ
- (2.2.5)
- if reversible : (2.2.6)
THERMODYNAMICS FUNDAMENTALS
- Heat transfer
- δQ differential form of the fluid mass state
- (2.2.7)
- δQ heat exchanged with the surroundings
- δπ heat dissipated by internal friction
Examples of calorimetric equations
For example, the state of a pure substance in liquid-vapor equilibrium at constant pressure exchanging heat dQ with the outside is governed by the equation:
dQ = L dx
L being the heat of vaporization of the fluid, and x is its vapor quality
Heating at constant pressure of a fluid is determined by the equation:
dQ = Cp dT
Cp being the heat capacity of the fluid, and T its temperature
THERMODYNAMICS FUNDAMENTALS
- First law: energy conservation
- closed system: internal energy
- (2.3.1)
- open system: enthalpy h = u + Pv
- (2.3.4)
THERMODYNAMICS FUNDAMENTALS
- Application to industrial processes
- (2.3.7)
THERMODYNAMICS FUNDAMENTALS
- Application to industrial processes
- heat exchangers: Q = Δh
- adiabatic machines: τ = Δh
- throttling: Δh = 0
First law of thermodynamics
This session showed that two forms of energy are involved in energy systems components: mechanical work of pressure forces and heat exchanged with the surroundings.
The different terms appearing in the expression of the first law in steady state, in closed or open system, were presented.
The sessions on components show how to evaluate these terms in practice.