Moist mixtures
A number of commonly used gases are mixtures whose composition may vary due to condensation or vaporization of one of their components. In practice, the component that changes state is mostly water, which justifies that a special chapter in this book is dedicated to mixtures of gases and water vapor, hereinafter referred to as moist mixtures.
In this page we limit ourselves to the calculation principles of thermodynamic properties of moist mixtures. While it is certain that the most numerous practical applications regard air, our presentation will cover all gases for which our assumptions are valid, that is to say the vast majority of cases. For example, the humidity properties of combustion products, which almost always contain water, may be determined by the methods presented here.
Definitions and conventions
When looking at a moist mix, we are dealing with a mixture of a gas that does not condense, which we call the dry gas, and water that could condense. Note that we will not discuss mist here; we can assume that the volume occupied by any condensed water is still very small compared to that occupied by the gas phase. We will therefore neglect mist. Under these conditions, for a given volume, the total mass of the gas phase can vary, while the mass of non-condensable constituents remains constant. Therefore it is customary to refer to the invariant mass which is the dry gas, all the thermodynamic properties of the mixture comprising the gas phase, the dry gas.
Everything happens as if somehow the moist mix was a mixture of two substances: the dry gas, whose composition is fixed, and water that may be present in one or more phases. As discussed later, it is essential, in order to carry out calculations, to clearly specify which substance the thermodynamic quantities used relate to, and what are the reference units and origin. The notations will be chosen accordingly.
We call specific humidity w the ratio of the mass of water contained in a given volume of moist mixture to the mass of dry gas contained in this volume.
The water in a moist mixture can be characterized in various ways: through its specific humidity, which we just introduced, but more typically by its mole or mass fraction, or by its partial pressure which is of particular interest here. As long as the partial pressure of water remains below its saturation pressure at the gas temperature, the water is in the form of vapor. Otherwise it exists at least partly in the condensed liquid or solid state. There is therefore an upper limit to the amount of water vapor that may be contained in a moist mixture. It depends on the temperature and pressure. When, at a given pressure, the temperature drops below the saturated vapor temperature of water, water vapor begins to condense as a mist or on cold walls that define the system if they exist. .
Given its practical importance, the saturated state is the reference, and we call relative humidity e the ratio of the partial pressure of water vapor divided by its saturation vapor pressure at the temperature of the mixture. This ratio is equal to 1 (or 100%) when the vapor begins to condense. Otherwise, it is less than 1.
We know that a condensable fluid remains comparable to an ideal gas at the immediate vicinity of the liquid state when its pressure is lower than its critical pressure. When this fluid is a mixture component, this rule applies taking into account the partial pressure.
As long as the liquid state does not appear, the mixture behaves like an ideal gas. When the mixture temperature drops below the condensation temperature corresponding to the partial pressure of the condensable component in the dry gas, liquid begins to appear. In most cases, although this is not strictly true, it is reasonable to consider that this liquid phase is pure, made up exclusively of the condensable component.
At that time, provided that equilibrium is established, experience shows that the situation is as follows:
the vapor-liquid equilibrium relationship is met by the condensable component, as if the other constituents did not exist. Its saturated vapor (index sv) partial pressure, given by the law of saturation pressure, is therefore Psv = Ps(T);
Dalton's law applies to the calculation of thermodynamic functions of the gas phase;
the law of phase mixture applies to the calculation of thermodynamic functions of the liquid and gas phases (assuming that the interfacial tensions are negligible).
To calculate the thermodynamic properties of the mixture in the presence of a liquid phase, the procedure is thus as follows:
knowing the temperature of the medium, one can determine the partial pressure of the condensable component. We deduce the sum of partial pressures (P - Psv) of the other constituents. As we know their relative molar fractions, we can completely determine the composition of the gas phase, and the number of moles of the condensable component in the gaseous state. By difference, we know the number of moles liquefied;
The Dalton and phase mixture laws are then used to calculate all the properties of the mixture. It is obvious that in this case the composition of the mixture changes as a function of the quality of the condensable component, and this must be taken into account in the calculation of thermodynamic properties.
In Thermoptim the calculation of moist mixtures is performed using this method. It is thus possible to study the behavior of a vapor and gas mixture of any composition.
Key Relationships
We will use indices dg to describe the dry gas, and mm for the moist mixture.
Case where the moist mixture composition is known
By definition of the partial pressure of water:
Pvap = xH2O . P (33)
The molar mass of the dry gas is:
Mdg = 1/(1 - xH2O)S Mnc xnc (34)
nc representing the various noncondensable constituents.
w = yH2O/ydg = MH2O xH2O / Mdg / xdg (35)
If Psv is the saturation vapor pressure of water:
If Pvap <= Pvs e = Pvap/Pvs
w = MH2O / Mdg . Pvap / (P - Pvap) (36)
If Pvap > Pvs e = 1
w = wsat = MH2O / Mdg . Pvs / (P - Pvs) (37)
The values of specific volume vspec and specific enthalpy q' can then be determined, the latter being calculated with an enthalpy zero at 0 °C.
Generally (and this is the case in Thermoptim) the reference state for calculating the gas enthalpies is taken at the standard value of 298 K. Now, HVAC engineers by custom choose 0° C as the reference state for dry air, and 0° C for saturated liquid water. The result is a mismatch between the enthalpies of moist gas as they are usually calculated and those represented on the usual psychrometric charts. This difference varies depending on the specific humidity.
In practice, as the enthalpies can be referred to three different references, you need to know how to distinguish them in order to avoid making mistakes. In what follows, we will use as appropriate:
the moist mixture enthalpy hmm (hmm = 0 à T = 298 K)
the dry gas enthalpy hdg (hdg = 0 à T = 298 K)
the moist mixture specific enthalpy q' (q' = 0 at T = 0 °C, liquid water)
In general, unless otherwise indicated, the first is used by default, whereas in the calculations specific to moist mixtures, it is usually the third form that is used.
In what follows, the index water indicates that the calculation is performed with the equations of water as a real fluid, and the index H2O that it is conducted with the equations of water treated as a perfect gas. hvwater is the enthalpy of water in the vapor state, and L0water represents the enthalpy of vaporization of water at 0 °C.
q'(t,w) = hdg(t) - hdg(0°C) + w hvwater(t, Pvap) (39)
or by making the approximation that water vapor behaves like an ideal gas:
q'(t,w) = hdg(t) - hdg(0 °C) + w [hH2O(t) - hH2O(0 °C)] + w L0water (40)
By introducing hmm, enthalpy of the (ideal) moist mixture:
hmm(t) = (hdg + w hH2O(t)) / (1 + w) we get:
q'(t,w) = (hmm(t) - hmm(0 °C)) (1 + w) + w L0water (41)
Similarly, vspec = vgh (1 + w)
In the supersaturated zone, the gas is saturated with water in the vapor state, the rest being water in the liquid state. The specific enthalpy is then given by::
q'(t,w) = hdg(t) - hdg(0°C) + w hvwater(t, Pvs)
Case where the dry gas composition is known
When we know the dry gas composition, the calculations are done slightly differently, because w is then given.
Pvap = P w Mdg / MH2O /(1 + w Mdg / MH2O) (42)
e = Pvap/Pvs (43)
Other calculations are carried out as before. .
Temperatures used for moist mixtures
The study of moist mixtures leads us to introduce several temperatures, whose definition it is important to know, because for a given state of the gas, their values may be substantially different..
We call dry-bulb temperature t (°C) or T (K), the temperature indicated by a thermometer whose sensing portion is completely dry, and is placed into the moist mixture. This is the temperature in the usual sense.
We call dew point tr (°C) or Tr (K) the temperature at which the first drops begin to appear when the moist mixture is cooled at constant humidity and pressure. If water condenses as ice, it is also known as ice temperature. This is the saturation temperature of the water at partial pressure Pvap.
With the previous notations, Tr = Tsat(Pvap) if Pvap £ Psv, and Tr = Tsat (Psv) = T, otherwise.
We call wet-bulb temperature t' (°C) or T' (K), the temperature indicated by a thermometer whose sensing element is covered with a thin film of water being evaporated due to strong mixing of the gas. In practice, the bulb of the thermometer is covered with a wick soaked with water. If measured in good conditions, this temperature is substantially equal to the adiabatic saturation temperature that would be obtained by moistening the gas to saturation in an adiabatic device, by spraying water at the temperature equilibrium.
One can show that its value is given by the following equation:
enthalpy of liquid water at t' (saturated moisture at t' - initial moisture) = = enthalpy of saturated mixture at t' - enthalpy of the initial mixture at t.
Relative to the dry gas, the equation is:
hlwater(t') (wsat - w) = hdg(t') - hdg(t) + wsat hvwater (t') - w hvwater(t) (44)
Relative to the specific units, it becomes:
q'(t', wsat) - q'(t,w) = (wsat - w) hlwater(t') (45)
This is an implicit equation in t', involving the mixture formed in part by the dry gas, and secondly by water. It can be reversed in a temperature range between a value close to the dew point as a minimum, and the dry bulb temperature as the maximum.
As stated above, we must take care that the reference values of enthalpies are consistent. Moreover, when t or t' falls below 0 °C, the equations of liquid water must be replaced by those of solid water (ice).
Moist mixture charts (Psychrometric charts)
The main thermodynamic relations that we have given show that moist mixture variables and state functions are connected by relatively complex equations, which justify seeking an easy to use graphical presentation.
Carrier and Mollier charts
Two broad families of charts exist: the charts derived from that proposed by Carrier, with the dry-bulb temperature as the abscissa and the specific humidity as the ordinate (left, Figure below), and Mollier charts (right, Figure below), with specific humidity as abscissa and the specific enthalpy as ordinate
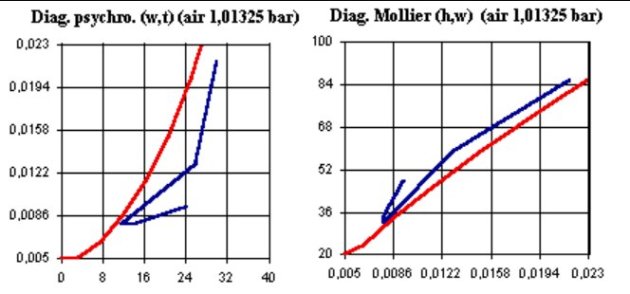
In the figure above, the gas water saturation curve is the smooth concave curve upwards in the Carrier chart (left) and downwards in the Mollier chart (right).
In the Carrier chart, the area above the saturation curve corresponds to cases where water is in excess and exists as a condensed liquid or solid: the gas is supersaturated. In the Mollier chart, the reverse is true: this zone is located below the saturation curve.
The charts in Figure above relate to air at atmospheric pressure. They are equipped with different iso-value curves:
curves of equal relative humidity concave upward;
isenthalpic curves are very close to straight lines and moist isotherm curves are almost parallel to them (usually not shown);
isovolume curves are very close to straight negative lines of slope stronger than the previous.
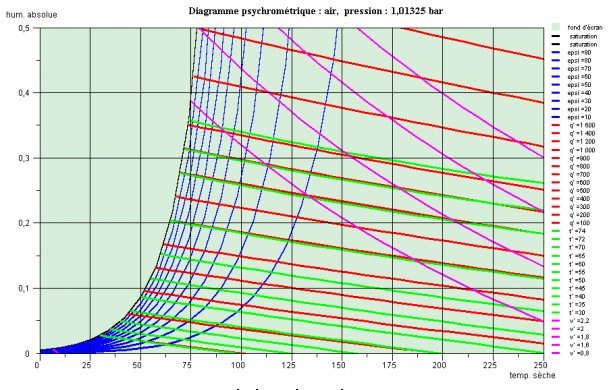
At atmospheric pressure and above 100 °C, it is impossible to condense water. On the Carrier chart, this translates into an almost vertical tangent on the saturation curve for 100 °C, and on the Mollier chart by an asymptote to the saturation curve, corresponding to dry isotherm 100 °C.
