Perfect and ideal gases
Many thermodynamic fluids in the vapor phase may be treated as ideal gases in a wide range of temperatures and pressures. This requires that the temperature-pressure combination deviates from the condensation zone as much possible (that is to say that the pressure is not "too" high or the temperature "too" low). Such conditions are commonly the case for gases known as "permanent" at ambient temperature and pressure, such as hydrogen, oxygen, nitrogen, the oxygen-nitrogen mixture that is dry air etc. Even the water vapor in the atmosphere behaves almost like an ideal gas as its partial pressure remains moderate.
The ideal gas model is based on the assumption that the molecular interactions in the gas can be neglected, except for collisions between them. The kinetic theory of gases can then explain the gas macroscopic behavior from mechanical considerations, and statistics on the movements of its molecules.
The fundamental assumption of ideal gases is that their internal energy (and their enthalpy) is independent of pressure. Given that all real gases can be liquefied, there is rigorously no ideal or perfect gas. These concepts are fundamental, however, because the practical determination of the state of a real fluid is always made by reference to the corresponding ideal or perfect gas, which approximates the behavior at very low pressure and/or high temperature.
Specifically, to represent the state of a fluid, a cascade of increasingly complex models is used depending on the desired accuracy, the simplest being that of the ideal gas, the most elaborate corresponding to real fluids.
Let us recall that Diapason session S04aEn is devoted to the properties of pure substances.
Equation of state of ideal gases
The equations of perfect and ideal gases are very close, the first being in fact a special case of the latter. The equation of state of an ideal gas can be written:
Pv = rT (1)
with r = R/M (kJ/kg/K)
R is the universal constant = 8,314 (kJ/kmol/K)
M is the molar mass of the gas (kg/kmol)
According to the units used, equation (1) takes different forms:
in mass units: P v = r T
in molar units: P vm = R T
Based on the total volume V occupied by the fluid, n being the number of kilomol:
in mass units: P V = m r T
in molar units: P V = n R T
We can prove that equation (1) implies in particular that the internal energy and enthalpy of an ideal gas depend only on its temperature, and that:
r = cp - cv
We thus have:
cv = du/dT (J/kg/K)
cp = dh/dT (J/kg/K)
We call a "perfect" gas an ideal gas whose specific heat capacities cp and cv are constant.
For such a gas, the internal energy and enthalpy are linear functions of temperature. Note that other authors call perfect gas what we call an ideal gas. In this case, they must each time tell whether the heat capacity of gas depends or not on the temperature.
The assumption of perfect gas (cp and cv constant) is rigorously met for monatomic gases (which have no rotation or molecular vibration mode The larger the number of atoms in the gas molecule (and thus possible vibration modes), the less this assumption is valid.
Statistical thermodynamics allows us to determine the values of molar heat capacity of monatomic and diatomic gases..
For the former, we get:
Cp = 5/2 R = 20.785 kJ/kmol Cv = 3/2 R = 12.471 kJ/kmol
For diatomic gases usually, at room temperature, we obtain:
Cp = 7/2 R = 29.1 kJ/kmol Cv = 5/2 R = 20.785 kJ/kmol
The figure below shows the changes in Cp for some typical mono-, bi-and tri-atomic gases.
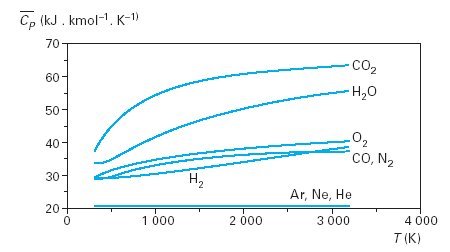
Molar heat capacity of some gases
Practical determination of the state of a perfect gas
Two parameters are sufficient to define an ideal gas: either its heat capacities at constant pressure and volume, or one of them and the value of its molar mass M, or the values of M and g, the ratio of cp to cv.
Under the assumptions, Pv = RT, cp - cv = r, and constant cp and cv, we can easily calculate the internal energy u, enthalpy h of the gas from any reference state T0.
u = u0 + cv (T - T0) et h = h0 + cp (T - T0) (2)
Tds = du + Pdv
ds = 1/T du + p/T dv
which allows us to easily calculate the entropy of the fluid: ds = cp/T dT + r/v dv
s = s0 + cp ln(P/P0) + cv ln(v/v0)
s = s0 + cv ln(T/T0) + r ln(v/v0) (3)
s = s0 + cp ln(T/T0) - r ln(P/P0)
The choice of the reference point is arbitrary and depends on conventions.
The characteristics (M, cp, cv, r, g) of some substances are given in the table below.
M
Cp
Cv
r
g
kg/kmol
J/kg/K
J/kg/K
J/kg/K
Air
28.97
1005
718
287.1
1.40
Hydrogen (H2)
2.016
14320
10170
4127
1.41
Helium (He)
4.003
5234
3140
2078
1.66
Methane (CH4)
16.04
2227
1687
518.7
1.32
Steam (H2O)
18.02
1867
1406
461.4
1.33
Neon (Ne)
20.18
1030
618
411.9
1.67
Acetylene (C2H2)
26.04
1712
1394
319.6
1.23
Carbon monoxyde (CO)
28.01
1043
745
296.6
1.40
Nitrogen (N2)
28.02
1038
741
296.6
1.40
Ethylene (C2H4)
28.05
1548
1252
296.4
1.24
Ethane (C2H6)
30.07
1767
1495
276
1.18
Oxygen (O2)
32.00
917
653
259.6
1.40
Argon (Ar)
39.94
515
310
208
1.67
Carbon dioxyde (CO2)
44.01
846
653
188.9
1.30
Propane (C3H8)
44.09
1692
1507
188.3
1.12
Isobutane (C4H10)
58.12
1758
1620
143.1
1.09
Octane (C8H18)
114.23
1711
1638
72.8
1.04
Practical determination of the state of an ideal gas
An ideal gas differs from a perfect gas because its thermal capacity is not constant, but depends solely on temperature.
Most often, Cp is represented by a polynomial fit of order n in T, as (either in molar units, as below, or in mass units):
Cp =
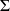 Cpi Ti (4)
Cpi Ti (4)
The solution chosen in Thermoptim is a 7-term development of the following type:
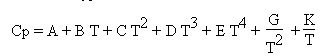
Cv is deduced by Cv = Cp - R, and specific variables cp and cv are obtained by dividing these values by the molar mass of the substance.
In practice, if we do not have a software application to calculate the properties of substances, and if we intend to determine the evolution of an ideal gas over a limited range of temperature, it is possible to assimilate it to a perfect gas, which enables us to use all the results established for them, provided its specific heat capacity is calculated at the mean temperature of the process. Of course, this is only valid in first approximation, but the loss of accuracy is compensated by a simplification of the calculations.
From a polynomial cp or cv = f (T), it is easy to calculate u, h or s by integrating their differential relations, which leads to:
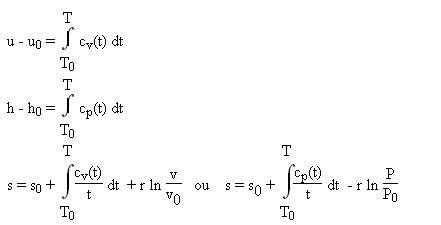
Equation of isentropic process
In many compressors or turbines, the fluid undergoes an evolution close to the isentropic, which forms the reference against which the actual process is calculated. The isentropic equations are therefore of particular importance.
By posing s = s0 = Const, we find, for perfect gas
or, in differential form:
dP/P +
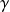 dv/v = 0 (9)
dv/v = 0 (9)
For an ideal gas, the isentropic equation does not take as simple a form as for a perfect gas. The formalism is more complex, but it remains quite usable in practice, as the pressure and temperature variables can be separated.
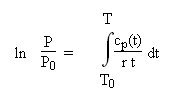
Ideal gas mixtures
In many practical applications, we are dealing not with pure gases, but gas mixtures, whose composition may vary. This is particularly the case in an internal combustion engine cylinder: the composition of flue gas is evolving gradually as the combustion unfolds.
Dalton's law states an important result: a mixture of ideal gases behaves itself as an ideal gas.
The composition of a mixture is usually determined from either mole fractions or mass fractions of the constituents. In this section we establish the expressions for various usual thermodynamic properties in terms of these quantities.
Mole fractions and mass fractions
The total number of moles n of the mixture equals the sum of the numbers of moles of each component:
n = n1 + n2 + n3 + ... nn =
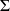 ni
ni
The mole fraction of a component is defined as the ratio of the number of moles of this constituent to the total number of moles in the mixture:
xi = ni/n and
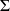 xi = 1
xi = 1
Moreover, the law of mass conservation implies that the total mass is equal to the sum of the masses of the constituents:
m = m1 + m2 + m3 + ... mn =
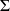 mi
mi
The mass fraction of a component is defined as the ratio of the mass of the component to the total mass of the mixture:
yi = mi/m and
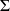 yi = 1
yi = 1
Dalton law of ideal gases
We define the partial pressure Pi of a component as the pressure exerted by this component if it occupied alone the volume V of the mixture, its temperature being equal to that of the mixture
Dalton's law postulates that the pressure, internal energy, enthalpy and entropy of a mixture of ideal gases at temperature T and pressure P are respectively the sum of the pressures, internal energies, enthalpies and partial entropies of gas constituents, that is to say taken separately at temperature T and their partial pressures.
Each component behaves as if it existed at the temperature T of the mixture and was alone in the volume V.
Physically, this means that the fields of molecular forces of the individual components do not interfere with each other.
Mathematically, Dalton's law translates into the following two laws, the total pressure being P:
Pi = xi P (10)
cp = 1/m
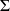 cpi mi =
cpi mi =
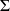 yi cpi (11)
yi cpi (11)
The mixture heat capacity at constant pressure equals the sum of the products of the specific heat capacity of the constituents by their mass fractions.
Dalton's law means that a mixture of ideal gases behaves itself as an ideal gas, whose fictitious mole molar mass would be M =
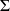 xi Mi.
xi Mi.
This means that the results established for ideal gases can be used to calculate the evolution of mixtures of these gases, which is of paramount importance in practice.
We have: yi = xi Mi/M
We can also define an equivalent ideal gas constant by:
r = R/M (kJ/kg/K)
In mass notations, the ideal gas constant of the mixture is expressed in a very simple form:
r =
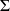 yi ri (12)
yi ri (12)
Moreover, H =
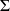 Hi et m cp T =
Hi et m cp T =
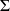 cpi mi T
cpi mi T
We deduce (11).
In molar notations, (11) becomes:
Cp =
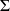 xi Cpi (13)
xi Cpi (13)
Energy properties of ideal gas mixtures
Enthalpy of a mixture
According to Dalton's law, the enthalpy of a mixture of ideal gases is equal to the sum of the enthalpies of each component.
mass notations: h =
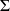 yi hi (14)
yi hi (14)molar notations: H =
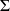 xi Hi (15)
xi Hi (15)
The calculation of internal energy would be done in the same way.
Entropy of a mixture
It is when calculating the entropy of the mixture that the profound significance of Dalton's law appears, i.e. that the value of the entropy of the mixture shall be calculated by summing the entropies of the constituents taken at temperature T and their partial pressures P i.
Indeed, the entropy of the mixture is greater than the sum of the entropies of the constituents before mixing by a value km = - r
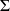 xi ln xi > 0. Physically, this is explained by the irreversibility of the mixing operation. It is important to note that the km gap remains constant (independent of T and P) as long as the concentrations do not vary. This gap must be taken into account once and for all when calculating the entropy of reference, but then, the mixture behaving itself as an ideal gas, we no longer need to worry about it.
xi ln xi > 0. Physically, this is explained by the irreversibility of the mixing operation. It is important to note that the km gap remains constant (independent of T and P) as long as the concentrations do not vary. This gap must be taken into account once and for all when calculating the entropy of reference, but then, the mixture behaving itself as an ideal gas, we no longer need to worry about it.
Of course, this does not remain true if the composition of the mixture comes to vary during the process, in which case it is necessary to calculate the different values of km to determine the variations of entropy.
Thermoptim uses two types of ideal gases:
pure gases, whose properties are predetermined in the software, not user-modifiable; they number about 20;
compound gases, constructed by the user at will from pure gases included in the database. Their properties are calculated by the software by applying Dalton’s law.
