Heat transfer to a fluid
This session discusses the thermodynamics of heat transfer to a fluid in an open system.
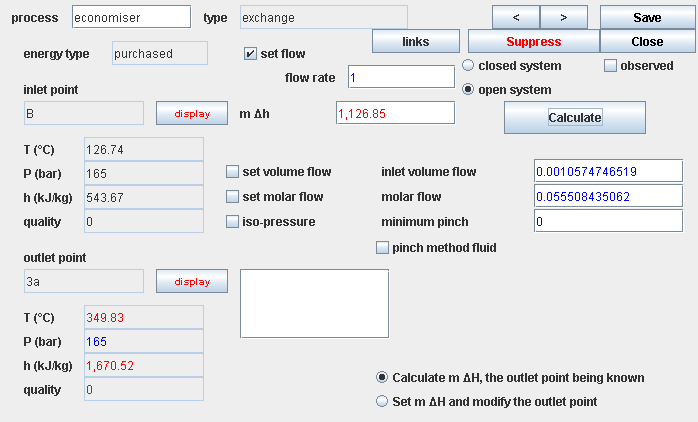
Components transferring heat to a fluid are the same type: a single fluid flows through them, exiting at a temperature different from that at the inlet. In most cases, we will assume that the outlet pressure equals the inlet pressure. If you want to refine the analysis, you can consider that there is a small pressure drop, but it may be neglected in first approximation.
It follows that the reference process is the isobar.
The organs in which heat transfer takes place being fixed, the shaft work involved is zero. Moreover, at the entrance and exit of these components, the velocities of fluids are always relatively low, so that the kinetic energies are negligible.
Under these conditions the first principle writes simply for heat exchangers: Δh = Q
Calculate the heat brought into play in these components is to determine the enthalpy change of the fluid flowing through them.
The calculation of a heat transfer being quite simple, we will express directly the two sequences of calculation steps depending whether the downstream point is known, or the amount of heat exchanged is known.
We will then present the Thermoptim screen for calculating heat transfers.
Course reference:
- “Technological Components / Heat Exchange”
To follow the presentation, go to the next step.
(Session realized on 06/16/11 by Renaud Gicquel)Heat transfer calculation (T2 known)
- calculate the inlet enthalpy: h1(P1, T1)
- calculate the outlet enthalpy: h2(P2, T2)
- calculate the enthalpy variation: Δh = h2 - h1
- calculate heat put in play: Q = m Δh
Heat transfer calculation (Q known)
- calculate the inlet enthalpy: h1(P1, T1)
- deduce the enthalpy variation: Δh = Q/m
- calculate: h2 = h1(P1, T1) + Δh
- reverse in T2: h(P2, T2) = h2
HEAT EXCHANGE 
Heat transfer to a fluid
This session discusses the thermodynamics of heat transfer to a fluid in an open system.
We showed that components transferring heat to a fluid are generally isobaric, and are crossed by a single fluid, exiting at a temperature different from that at the inlet.
In session S13En, we show how two “exchange” processes, one that represents a fluid which is heated and the other a fluid that cools, can be combined to form a heat exchanger.