Properties of substances, usual charts
This session is an introduction to the properties of substances which briefly presents the usual charts (entropy (T, s) and refrigeration (h,P)). Some guidance on the calculation of real fluids, and in particular on the properties of a mixture of phases in liquid-vapor equilibrium is also given.
The link below gives you access to an excerpt of the textbook presenting the properties of substances, to which you should refer.
Course reference:
- “Thermodynamics fundamentals / Representation of substance properties”
To follow the presentation, go to next step
(Session realized on 06/15/11 by Renaud Gicquel)STATES OF MATTER
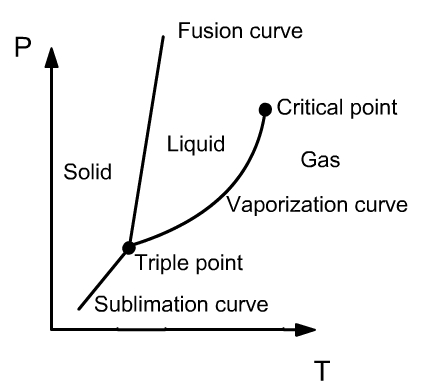
- Three phases
- solid
- liquid
- gaseous
STATES OF MATTER

- Saturation curves
- fusion
- vaporization
- sublimation
Fluid properties
- Ideal gas
- Real fluids (condensable)
Ideal gas model
- molecular interactions in the gas can be neglected
- internal energy (and enthalpy) independent of pressure
Ideal gas equation of state

- Pv = rT
- r = R/M
- R =8.314 kJ/kmol/K
- M molar mass
- r = cp - cv
Dalton’s law
- A mixture of ideal gas behaves itself as an ideal gas
- Each component behaves as if it existed at the temperature T of the mixture and was alone in the volume V
Practical determination of the state of a perfect gas
- Two parameters
- and
- or and
- and
Practical determination of the state of an ideal gas
- Ideal gas
- (2.6.4)
- (2.6.5)
Practical determination of the state of an ideal gas
- Ideal gas
- (2.6.6)
- (2.6.7)
Practical determination of the state of an ideal gas
- isentropes
- (2.6.9)
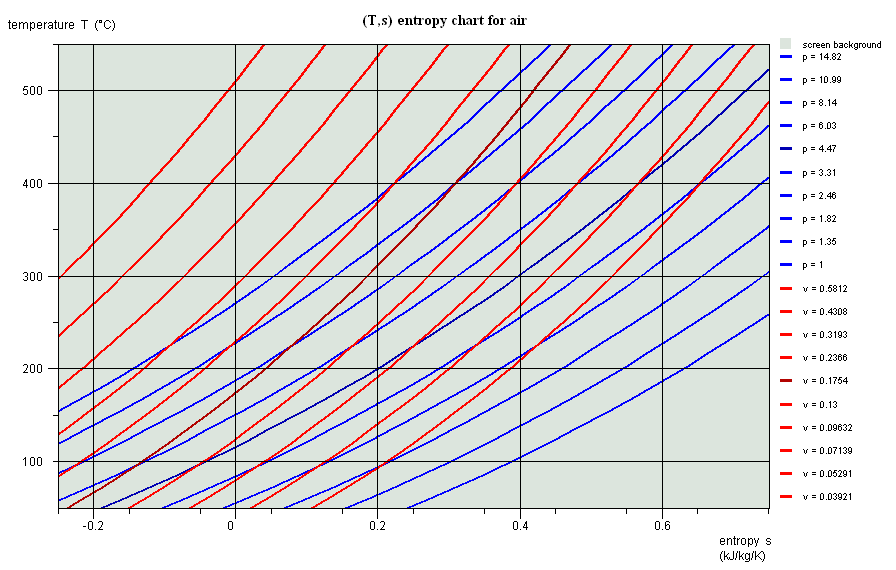
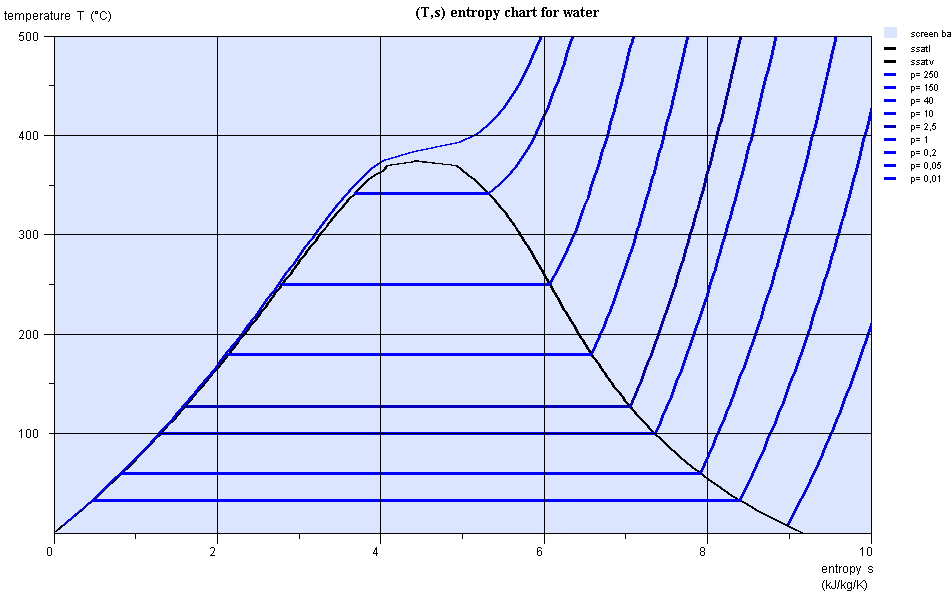
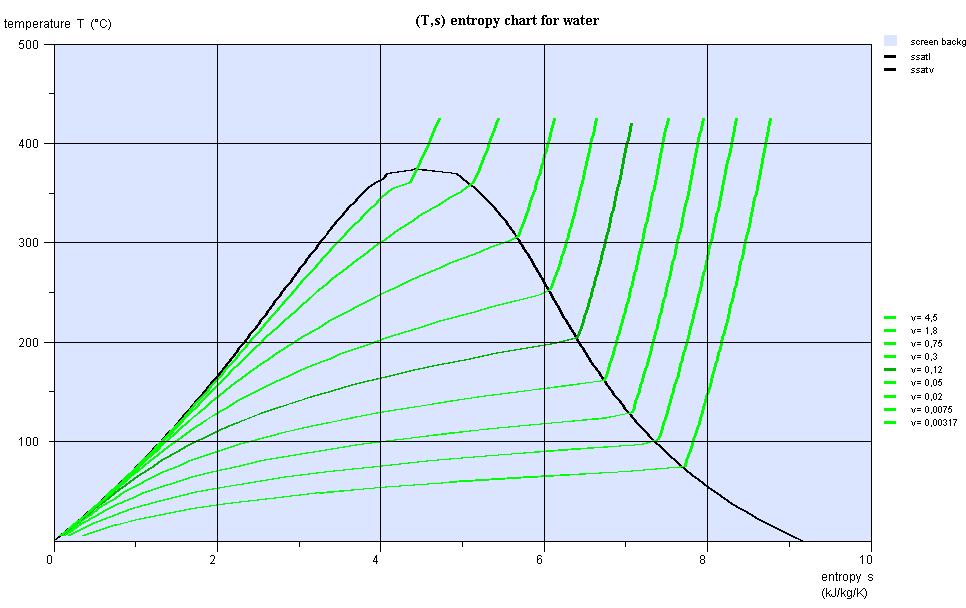
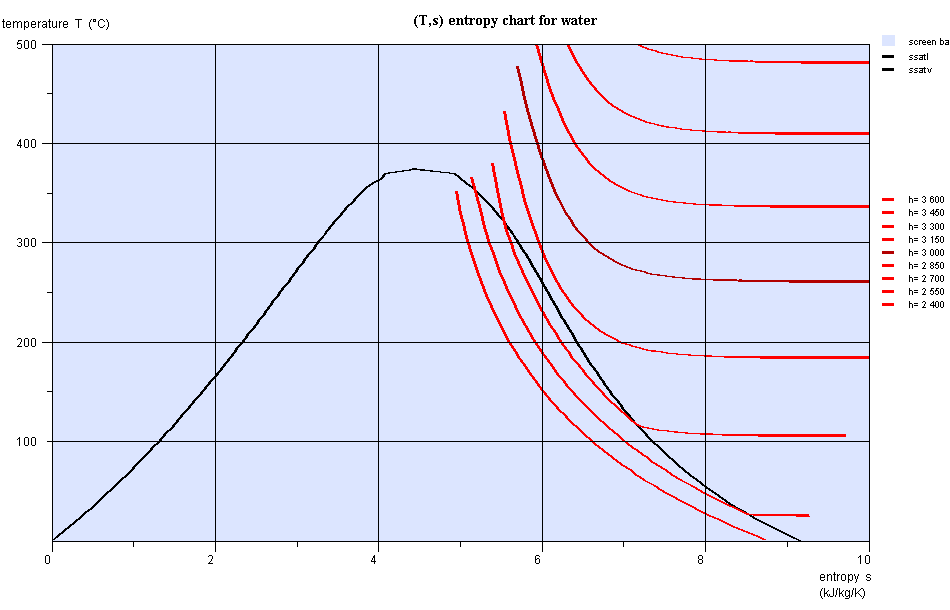
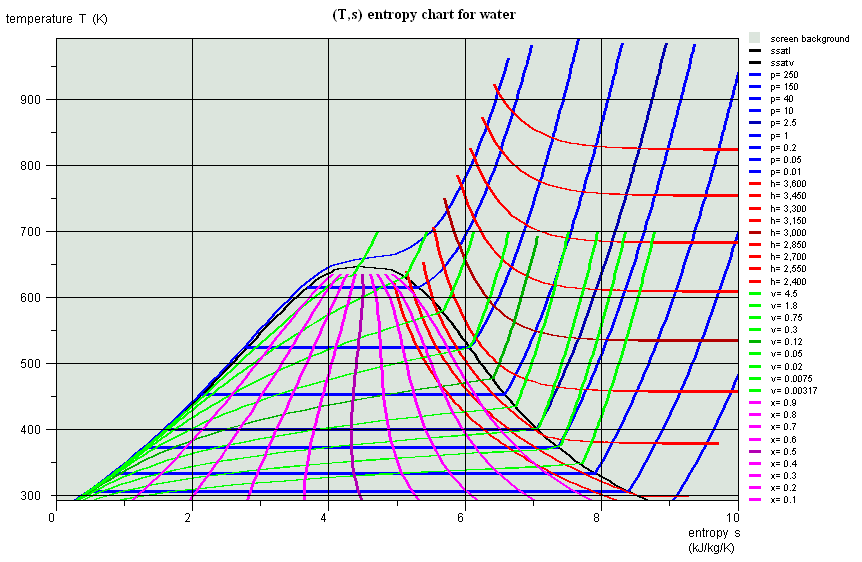
Entropy chart (T,s)
- area measures the absolute value of the amount of heat Q put into play or the work provided or received τ
Entropy chart (T,s)
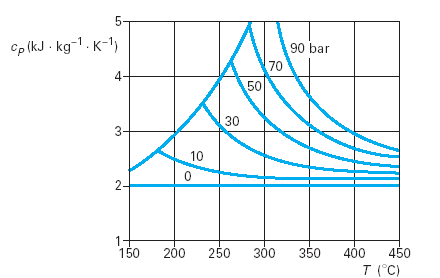
Properties of real fluids
- Real fluids
- Cp = f(T,P)
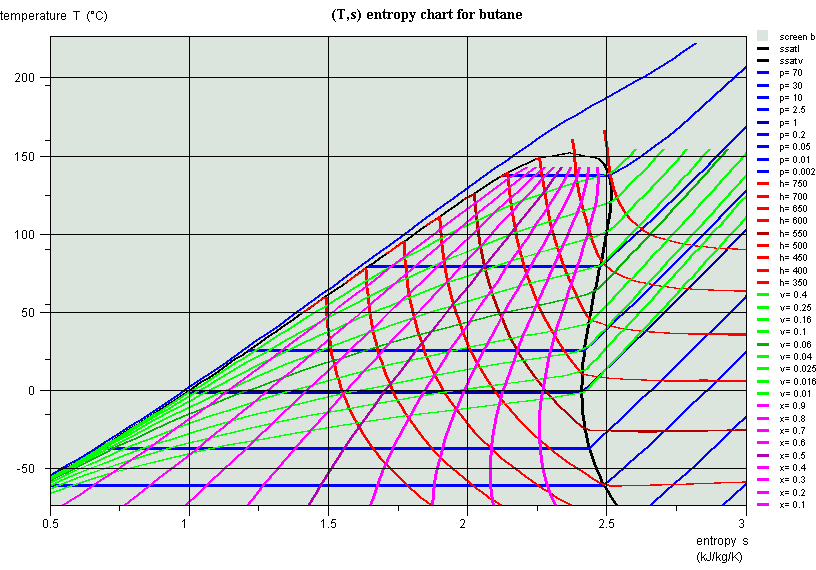
Liquid-vapor equilibrium
- quality
- law of phase mixture
Entropy chart (T,s)

Entropy chart (T,s), butane
Entropy chart (T,s), water
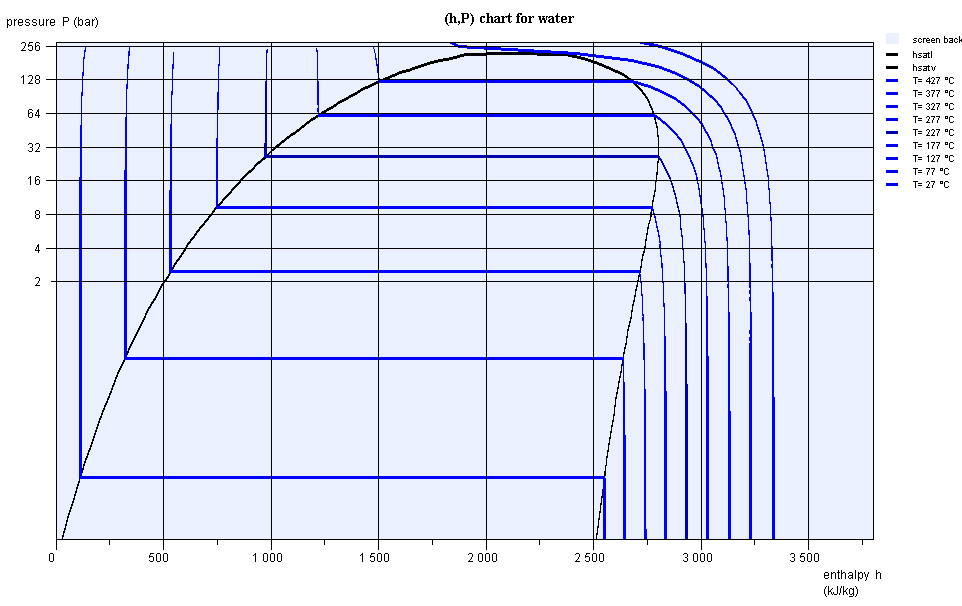
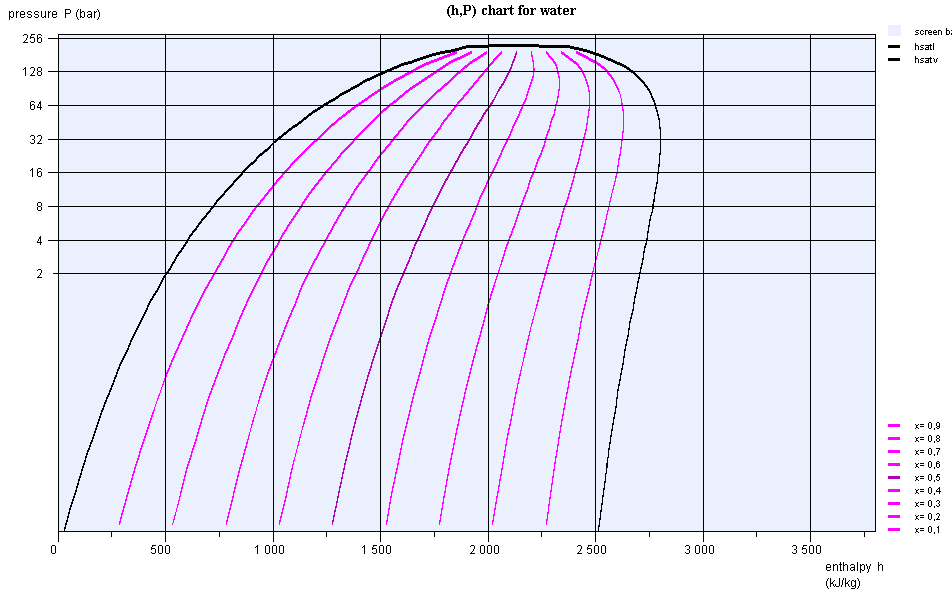
(h,ln(P)) chart


(h,ln(P)) chart, R134a
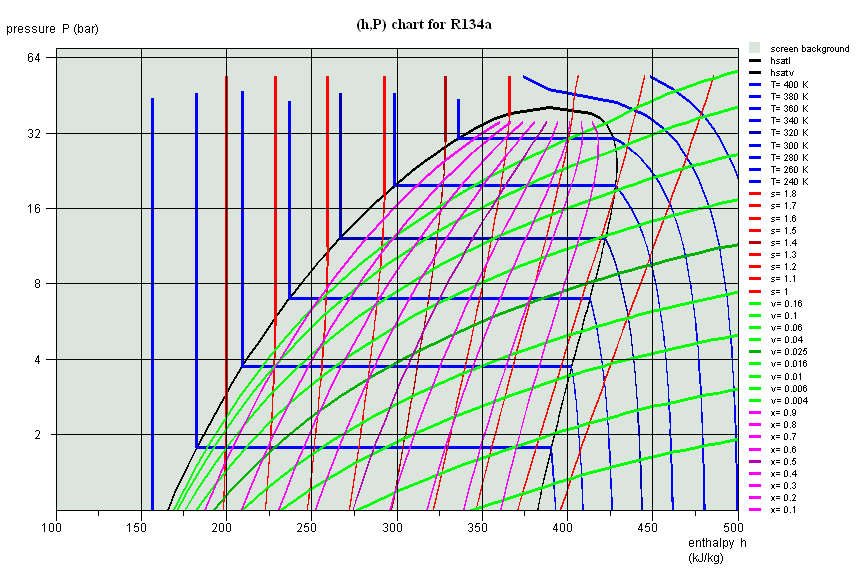
(h,ln(P)) chart, ammonia
Properties of substances, usual charts
This session showed how to calculate the properties of a simple or compound ideal gas, and for real fluids, presented the entropy (T,s) and refrigeration (h, P) charts are the most used in practice.